Suganon / Evergreen Clincs< Come back
Evergreen Study10
Evogliptin is a new once-daily DPP4 inhibitor that promotes up to 2.4 times increase in active GLP1, resulting in a postprandial glycemic reduction of up to 35% compared to placebo8. In addition, evogliptin does not require dose adjustment for patients with mild to moderate renal failure11.
The objective of EVERGREEN10 study was to evaluate the efficacy, through the reduction of HbA1c and the modification of parameters of continuous glucose monitoring (CGM) and the safety of evogliptin, using linagliptin as an active control. In this double-blind, multicentric study, 207 patients with type 2 diabetes mellitus with HbA1c between 7.0 and 10.0% were randomized 1:1 to treatment with evogliptin 5 mg/day (n-102) or linagliptin 5 mg/day (n« 105) for 12 weeks, followed by an extension period of an additional 12 weeks.
After 12 weeks of treatment, mean HbA1c reductions of -0.85% and -0.75% were observed in the groups of individuals treated with evogliptin 5 mg/day and linagliptin 5 mg/day, respectively (Figure 4).
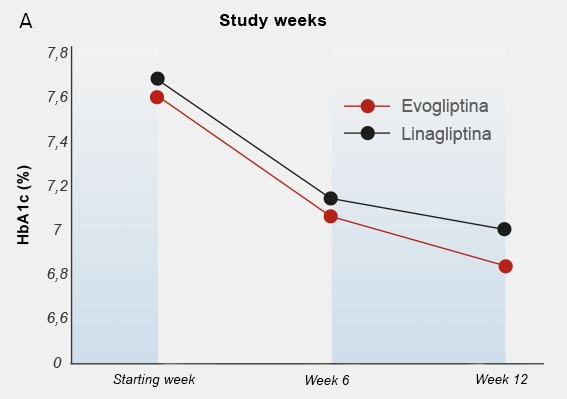
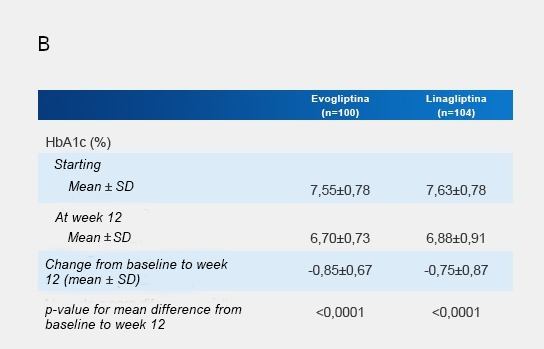
Fig. 4: A) Change in HbA1c (%) from initial week to week 12 of treatment. B) Mean HbA1c values (%) at baseline and after 12 weeks of the study in the type 2 diabetic patient groups treated with evogliptin 5 mg once/daily and
In the extension period (12 weeks following the end of the treatment period), evogliptin 5 mg/day was administered to both treatment groups: individuals who received evogliptin 5 mg/day in the treatment period maintained medication and individuals who received linagliptin 5 mg/day were switched to evogliptin 5 mg/day. Treatment with evogliptin resulted in a sustained reduction in HbAlc. The change in HbAlc from baseline to week 24 is shown in Figure 5.
Safety assessment results showed that the frequency of adverse events was similar in both treatment groups. In particular, the evogliptin group had no documented symptomatic hypoglycemia throughout the study period.
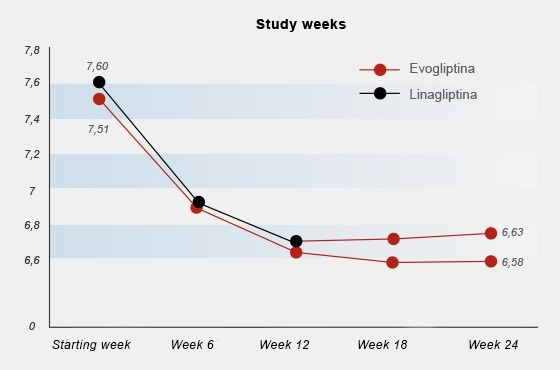
Fig. 5: Change in HbA1c (56) from initial week to week 24 of treatment. From week 12 of the study, participants who were using linagliptin 5 mg once/daily were switched to evogliptin 5 mg once/daily. Participants allocated to the evogliptin 5 mg once/daily group at the beginning of the study (initial week) maintained the use of evogliptin until week 24. Adapted from: Kim G. et al. Da betes Obes Metab. 2020 Sep;22(9): 1527-153610
Evogliptin 5 mg once daily is non-inferior to linagliptin and offers a new
option for treating type 2 diabetes mellitus10.






















